PulsBioInk
Development and characterization of a novel bioink for fabrication of 3D printed bioartificial pulsatile prosthesis for applications in tissue engineering – PulsBioInk
The aim of the project is to establish conditions for development of mechanically stable 3D printed stents which will be act as biocompatible biomimetic constructs containing human cardiomyocytes and endothelial cells.
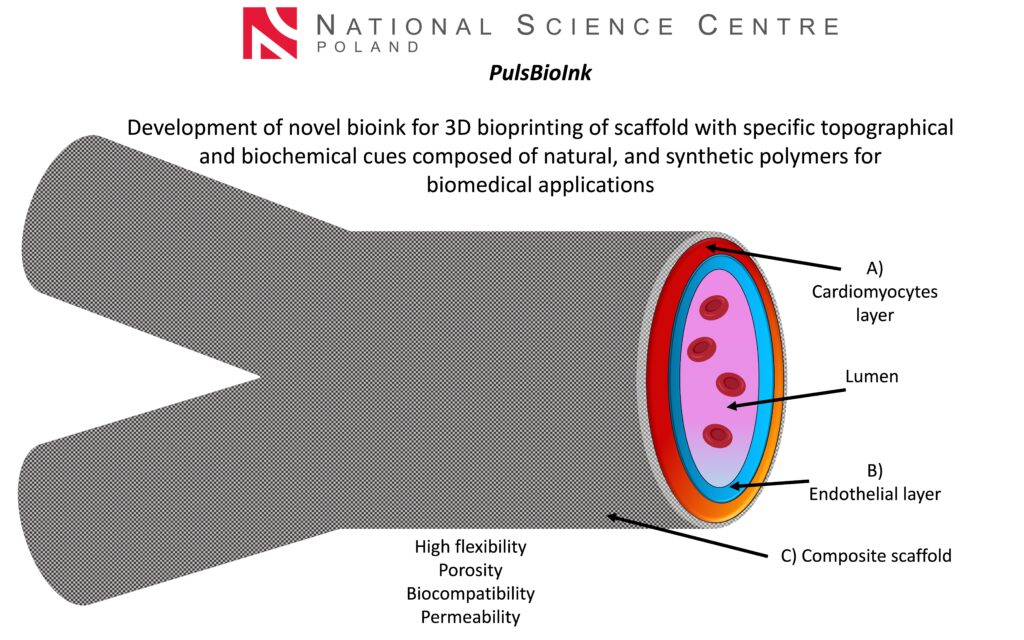
The main goal of the Sonata PulsBioInk project is to produce novel innovative bioimplants using the highly reproducible 3D bioprinting technique. Natural polymers based on silk fibroin, which possess unique biological and mechanical properties, biodegradability, biocompatibility, and bioresorbability, are used in combination with synthetic polymers with specific fiber architecture to improve the stability and mechanical properties of the scaffolds. The detailed impact of 3D-bioprinted grafts on encapsulated cell behavior as well as interactions between two types of human cells (cardiomyocytes and endothelial cells) are studied in vitro and in a designed ex vivo bioreactor system. Bioartificial prosthesis can be easily adopted to patient derived iPSCs- cardiomiocytes (iPSCs-CMs) and endothelial cells (iPSCs-ECs).
Cardiovascular diseases (CVD) are one of the leading causes of death worldwide, leading to 17.9 million deaths each year. CVD is a general term covering a wide range of disorders of the heart and blood vessels that most commonly affect people over the age of 60. Congenital heart disease (CHD) is one of the causes of chronic CVD, which is the most common cause of congenital pathologies and the most common congenital malformation, affecting almost 1% of all live births. In 2019, CHD was the leading cause of 217,000 deaths, of which 150,000 deaths were in infants under 1 year of age. A quarter of children affected by coronary heart disease will require major reconstructive surgery in their lifetime. Although significant improvements have been made in the treatment of congenital heart defects in recent decades, they remain the leading cause of death in the neonatal period. In the treatment of CHD, grafts made of synthetic materials such as polytetrafluoroethylene (PTFE or Gore-Tex) are used, which are prone to strictures, thromboembolism and infections. Graft failure rates have been reported to be 70 to 100% over 10 years. Therefore, patients require a series of reoperations to replace failed grafts, each of which is associated with mortality. Vascular tissue engineering provides a potential solution to these limitations.
The project aims to produce a highly stable for long-term culture cellular prosthesis construct that can be used as a model for testing/or treatment of cardiovascular diseases in the future.
Principal Investigator